
 Berkeley - October 1, 1999 - If experiments at the University of California, Berkeley, are any indication, future explorers of our solar system may well find diamonds hailing down through the atmospheres of Neptune and Uranus.
Berkeley - October 1, 1999 - If experiments at the University of California, Berkeley, are any indication, future explorers of our solar system may well find diamonds hailing down through the atmospheres of Neptune and Uranus.These planets contain a high proportion of methane, which UC Berkeley researchers have now shown can turn into diamond at the high temperatures and pressures found inside these planets.
"Once these diamonds form, they fall like raindrops or hailstones toward the center of the planet," said Laura Robin Benedetti, a graduate student in physics at UC Berkeley.
The team, led by Benedetti and Raymond Jeanloz, professor of geology and geophysics, produced these conditions inside a diamond anvil cell, squeezing liquid methane to several hundred thousand times atmospheric pressure. When they focused a laser beam on the pressurized liquid, heating it to some 5,000 degrees Fahrenheit, diamond dust appeared.
They report their experimental findings in a paper in the Oct. 1 issue of Science.
The demonstration that methane can convert to diamond as well as other complex hydrocarbons in the interiors of giant planets like Neptune hint at a complex chemistry inside gaseous planets and even brown dwarf stars. Brown dwarfs are small, dim stars barely larger than the largest gas giant planets.
"This is opening the door to study of the interesting types of chemical reactions taking place inside planets and brown dwarfs," Jeanloz said. "Now that technology is able to reproduce the high pressures and temperatures found there, we are getting much better quality information on the chemical reactions taking place under these conditions."
"It is not amazing that chemistry like this happens inside planets, it's just that most people haven't dealt with the chemical reactions that can occur," Benedetti said. "The interior of these planets may be much more complicated that our current picture."
A simple calculation, for example, shows that the energy released by diamonds settling to the planet's core could account for the excess heat radiated by Neptune, that is, the heat given off by Neptune in excess of what it receives from the sun.
"What's exciting to us is the application of this high-pressure chemistry to understanding the outer planets," Jeanloz said.
"As more planets are found in unexpected orbits around other stars, the effects of internal chemical processes will need to be further clarified in order to obtain a general understanding of planet formation and evolution," the authors concluded in the Science paper.
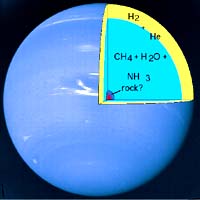 Our solar system's other gas giant planets -- Jupiter and Saturn -- may also contain diamonds produced under such conditions, though they contain proportionately less methane than Neptune and Uranus. Based on theoretical calculations, Neptune and Uranus are estimated to contain about 10 to 15 percent methane under an outer atmosphere of hydrogen and helium. (See graphic for presumed internal structure of Neptune,
Our solar system's other gas giant planets -- Jupiter and Saturn -- may also contain diamonds produced under such conditions, though they contain proportionately less methane than Neptune and Uranus. Based on theoretical calculations, Neptune and Uranus are estimated to contain about 10 to 15 percent methane under an outer atmosphere of hydrogen and helium. (See graphic for presumed internal structure of Neptune,Several groups of researchers have suggested that the methane in these planets could conceivably turn into diamond at fairly shallow depths, about one tenth of the way to the center. Nearly two decades ago, a group at Lawrence Livermore National laboratory shocked some methane and reported the formation of diamond before the stuff evaporated. That group was led by retired scientist Marvin Ross and researchers William Nellis and Francis Ree.
Recently some theorists in Italy also concluded that diamonds were likely.
Benedetti and Jeanloz decided to try the obvious experiment -- squeeze liquid methane and see if they could make diamond dust.
The liquid methane, cooled with liquid nitrogen, was placed in a diamond anvil cell and squeezed to between 10 and 50 billion pascals (gigapascals), or about 100,000 - 500,000 times atmospheric pressure. The researchers then heated the compressed methane with an infrared laser to about 2,000 to 3,000 Kelvin (3600-5400 degrees Fahrenheit).
"It's really cool to watch," said Benedetti. "When you turn on the laser the methane turns black because of all the diamonds created. The black diamond specks float in a clear hydrocarbon liquid melted by the laser."
Raman spectroscopy confirmed the identity of the suspended specks, as did subsequent analysis with X-ray crystallography. The flecks were diamonds interspersed with hydrocarbons.
Jeanloz said that the high temperature breaks up methane (CH4) into carbon and hydrogen, while high pressure condenses the carbon to diamond. Other types of hydrocarbons -- doubly and triply bonded carbon -- also were produced, apparently in the cooler areas outside that illuminated by the laser.
Jeanloz and his team plan next to see what happens to other constituents of these planets -- ammonia and water -- at high temperatures and pressures.
Coauthors of the paper with Benedetti and Jeanloz are post-doctoral researcher Jeffrey H. Nguyen, now a scientist at Lawrence Livermore National Laboratory; geology graduate student Wendell A. Caldwell, Chinese visiting scholar Hongjian Liu and Michael Kruger, a former graduate student now in the physics department at the University of Missouri, Kansas City.
No comments:
Post a Comment